/ Mareana's Breakthrough
Manufacturing Intelligence,
Reimagined.
Mareana’s GxP-compliant platform transforms manufacturing data into insights and outcomes — optimizing production, accelerating releases, and ensuring quality.
Ready for a deeper dive?
By submitting your details, you agree to our Privacy Policy, and to receive email communications from Mareana. You may unsubscribe at any time.
/ Unlock Your Edge
Key Benefits
Mareana has helped numerous firms in the Pharmaceutical, Chemical, Medical Device, and Industrial Manufacturing industries achieve significant business outcomes:

Optimize Production
- Increase Yield
- Shorten Cycle Time
- Minimize Rejection Rate

Accelerate Releases
- Streamline Batch Review
- Improve Right First Time (RFT) Rate
- Expedite Deviation Resolution Time

Ensure Quality
- Reduce Deviation Occurrences
- Optimize CAPA Closure Rate
- Minimize OOS Incidents
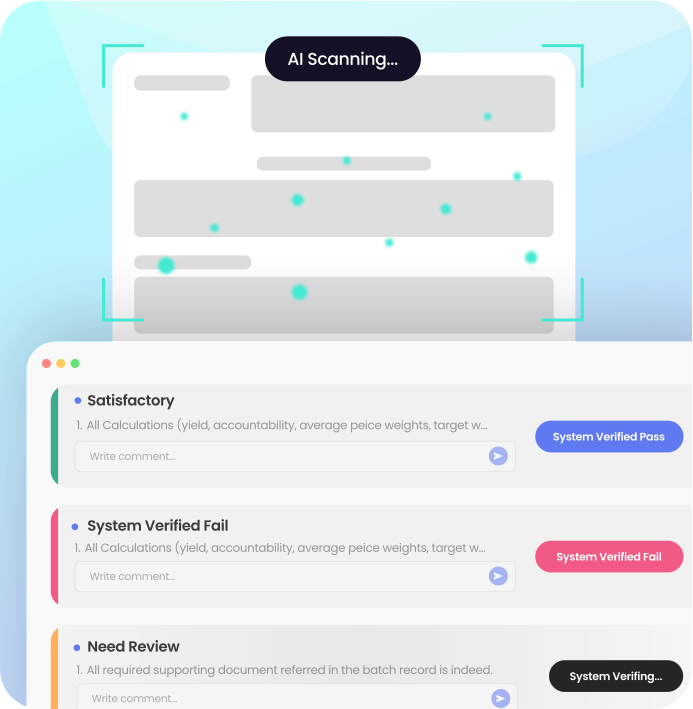
/ BATCH RELEASE COPILOT
AI-Powered Review By Exception
Batch Release Copilot uses AI to automate routine batch record checks, identify exceptions, and intelligently routes them to quality teams for review, ensuring compliant and efficient batch releases.


Good Manufacturing Practice
Connected Data Deep Insights.
/ AI AUTOMATION
GxP By Design
Mareana makes compliance effortless, going beyond industry standards to support GxP best practices. With built-in validation protocols, we take the heavy lifting off your plate, delivering secure access, e-signatures, and audit trails right out of the box.
-

Software Development
Mareana follows an SDLC process that has been audited by the largest life sciences brands in the world.
-

Installation & Configuration
We make it simple to validate and maintain with vendor-supplied IQ, OQ, PQ protocols.
-

Change Controls
Use of multi-tiered environment for major changes and patches.
-

21 CFR Part 11 & EU Annex 11
Mareana delivers compliant capabilities out of the box like secure user access, e-signatures, and audit trails.

Good Manufacturing Practice
Connected Data Deep Insights.
/ ANY QUESTIONS? WE’D LOVE TO HELP
Frequently Asked Questions.
Mareana helps you modernize without disruption, reduce QA burden, and prepare every site for faster approvals. Our pre-built pharma data models bring manufacturing, quality, and lab data together out of the box.No custom development. Just visibility that drives smarter decisions, continuous improvement, and faster regulatory responses.









