/ DATA INTEGRATION & BEYOND
AI-Powered
Manufacturing Intelligence
Go from data overload to decisive action. We harmonize your complete manufacturing landscape, from legacy paper to real-time process data, to accelerate batch releases, predict quality events, and fortify compliance at every stage.
Ready for a deeper dive?
By submitting your details, you agree to our Privacy Policy, and to receive email communications from Mareana. You may unsubscribe at any time.
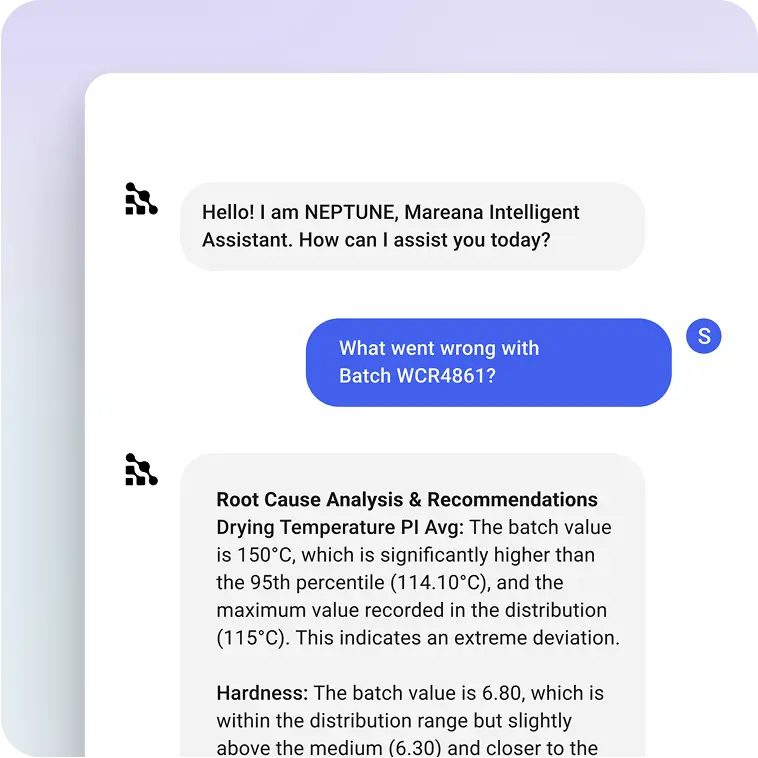
/ Neptune
Chat With Your Manufacturing Data
Explore your manufacturing data using natural language. Ask questions, surface insights, investigate deviations, and even generate charts. It's like having a data scientist, historian, and process expert in one conversational interface.

Accelerate Investigations

Uncover Hidden Insights

Ask complex questions in plain English

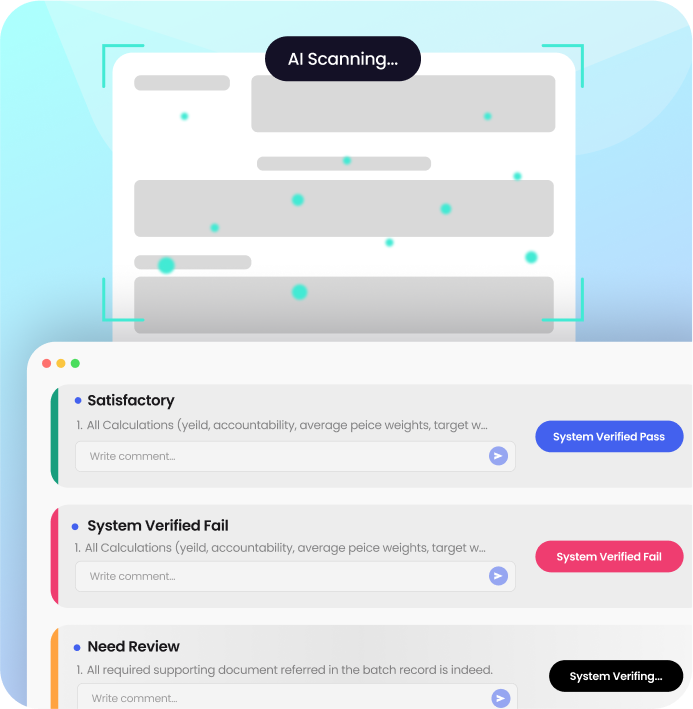
/ Batch Release Copilot
AI-Powered Review by Exception
Batch Release Copilot transforms your most significant compliance bottleneck into a strategic advantage. Our AI-assisted solution turns manual batch record review into an intelligent, exception-only workflow, empowering your team to release products days faster while minimizing errors and guaranteeing compliance.

Accelerate time-to-market for critical products

Identify deviations a human eye would miss

Focus expert judgement only on true exceptions
/ AUTO-APQR
The End of APQR Season
Automate your Annual Product Quality Review using harmonized data from across every system. With a single click, generate a compliant, audit-ready APQR (complete with trend analysis and deviation categorization), freeing your team from months of manual work.


Slash APQR generation time from months to hours.

Identify quality trends proactively, not retrospectively.

Liberate your experts for strategic quality initiatives.

And there’s more...
Mareana has all the tools you need to optimize production, accelerate releases, and ensure compliance.
Genealogy
Transform fragmented manufacturing data into a complete, visual batch history, enabling instant recall response, simplified investigations, and guaranteed GxP compliance.
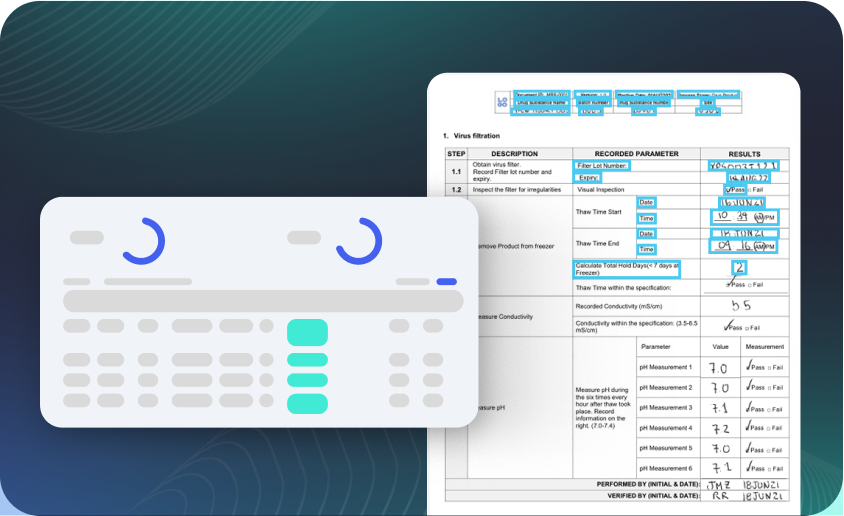
Paper Batch Record Digitization
Unlock legacy data from paper to accelerate batch release and ensure GxP compliance.
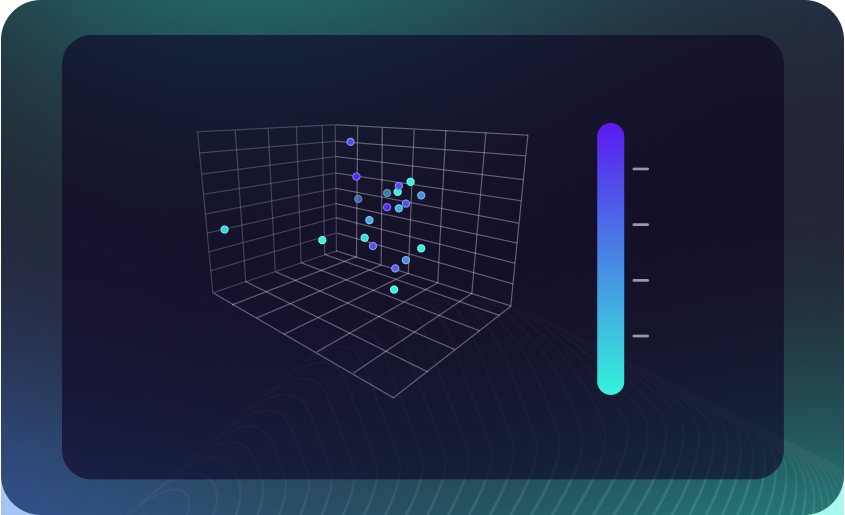
Data Science Studio
AI-powered analytics and no-code ML for immediate insights, optimizing manufacturing processes.
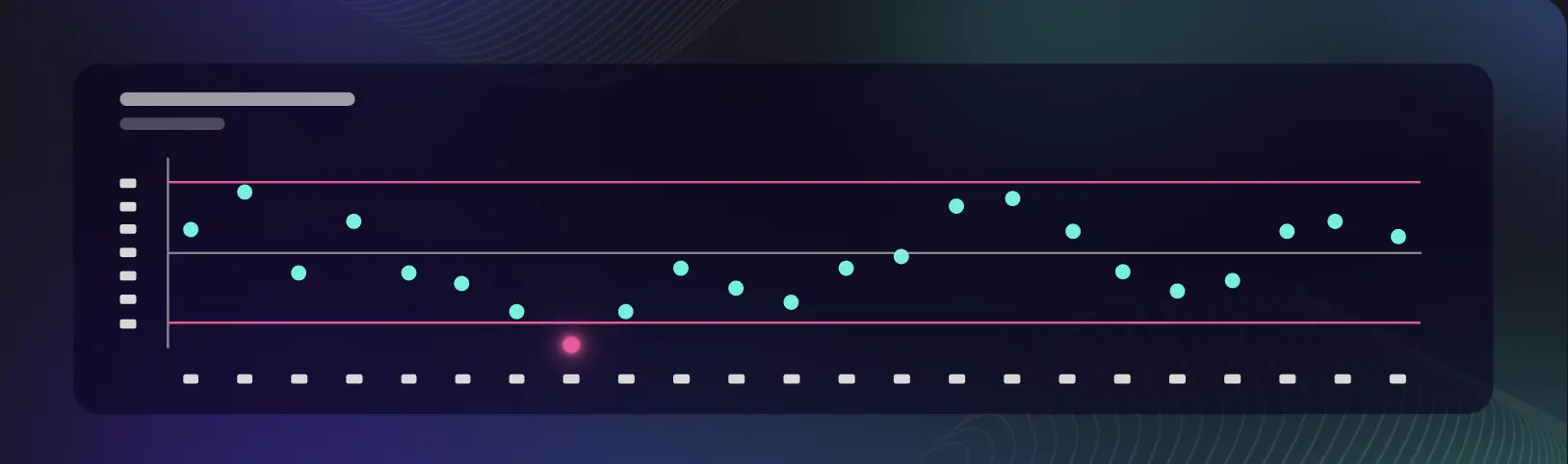
Smart CPV
Anticipate quality deviations before they impact your batch, using predictive analytics to reduce investigation costs, ensure compliance, and protect product quality.
/ MODERN PHARMA VISIONARIES
Who is Mareana for?






/ A FRAMEWORK FOR TRUST
GxP by Design
Mareana makes compliance effortless, going beyond industry standards to support cGxP best practices. With built-in validation protocols, we take the heavy lifting off your plate, delivering secure access, e-signatures, and audit trails right out of the box.
-

Software Development
Mareana follows an SDLC process that has been audited by the largest life sciences brands in the world.
-

Installation & Configuration
We make it simple to validate and maintain with vendor-supplied IQ, OQ, PQ protocols.
-

Change Controls
Use of multi-tiered environment for major changes and patches.
-

21 CFR Part 11 & EU Annex 11
Mareana delivers compliant capabilities out of the box like secure user access, e-signatures, and audit trails.

/ LATEST CASE STUDY AND BLOGS
Mareana Resources
Life sciences manufacturing best practices, original research, news and more