Manual batch record review in pharma is slower and riskier than leaders realize. The process was designed for a paper-based world where data was scarce, but today’s manufacturing generates massive volumes of complex, contextual data that overwhelm human reviewers. Reviewer fatigue, hidden handwritten notes, missed calculations, and “invisible” missing data (like absent signatures) create real compliance and inspection risks.
Review by Exception (RbE) modernizes quality assurance by shifting reviewers away from line-by-line checking of clean data and toward focused evaluation of true anomalies. Powered by modern AI, RbE digitizes paper records, automatically verifies rules, detects unstructured risks (margin notes, strikeouts), and creates a clear audit trail. Platforms like Mareana make RbE practical today, reducing batch review time by up to 70%, improving right-first-time outcomes, and providing regulators with objective, traceable evidence. In short: trusting manual review alone is now the bigger risk.
In the pharmaceutical industry, it is an open secret that batch record reviews are a massive operational bottleneck. They are notoriously time-consuming and are prone to human error due to reviewer fatigue and the sheer volume of verifications involved.
There is a common pattern in many mature industries: systems evolve faster than the assumptions behind them. In pharmaceutical manufacturing, we are modernizing how data is captured, stored, and retrieved, but batch review decisions are still largely based on a model designed for a paper world. That model worked well when data was scarce and variability was visible. Today, data is abundant, and risk often hides in context rather than values. Review by Exception is not a rejection of traditional QA judgment, rather it is an adaptation of it to modern scale and complexity.
The concept of Review by Exception (RbE) has long been the “holy grail” for speeding up this process, yet its implementation has remained challenging. For years, traditional OCR technology struggled with the variability of handwritten notes and complex paper templates, often creating more work than it saved. Other factors related to governance, validation, trust, risk, etc. also made OCR unreliable for batch reviews.
However, as AI technology has matured, the game has changed. Today’s AI can read handwritten paper records with high accuracy and analyze them to ensure they are not just legible, but correct.
Here is how Mareana’s Manufacturing Intelligence Platform is leveraging AI to make Review by Exception a reality for modern pharma.
What is Review by Exception (RbE)?
Review by Exception is a quality assurance strategy where reviewers only examine data that deviates from predefined standards. Instead of line-by-line audits of “clean” data, the system automatically flags errors, allowing Quality Assurance (QA) teams to focus their expertise where it matters most: on the anomalies. By eliminating the habit of rechecking clean data just to ‘feel safe,’ RbE shifts the workflow from simple omission to strategic focus

Why do Pharma Manufacturers need Review by Exception
Let’s understand the current challenges with an example:
A batch record with 500 pages and 68 parameters (CMA, CPP, CQA) is being reviewed late in the day. All values are within range, calculations are correct, and no deviations are logged. The reviewer re-reads multiple sections not because anything is wrong, but because they feel confident only after they’ve had multiple re-checks.
Buried in the margin notes is a handwritten note explaining a minor timing adjustment, signed and justified correctly. Like “Hold time extended by 15 minutes due to line clearance delay. OK per SOP”. In manual review, let’s even assume that the reviewer sees this and thinks, “let me check”. He takes a mental call that the SOP allows this.
Months later, an inspector asks, “How do you ensure hold time extensions are consistently evaluated?”. The site head says, “Reviewers check during batch review.” The Inspector says “Show me where this was evaluated”. You know where this goes.
How Review by Exception Changes the Narrative
Now, consider the same scenario under a Review by Exception model. The 500-page record is no longer a daunting haystack for a human to sift through; it is a structured data set managed by a digital partner.
When the system encounters that same handwritten margin note—“Hold time extended by 15 minutes due to line clearance delay”—it doesn’t just “see” it; it evaluates it against the validated logic of your process.
Instead of a reviewer making a subjective “mental call” late in the day, the Mareana platform executes a predefined hierarchy of checks:
- The Limit Check: The system cross-references the Master Batch Record (MBR) and SOPs to verify if an extension of ≤ 15 minutes is within the validated threshold.
- The Condition Check: It automatically scans the record to ensure the required condition in this case, the documented line clearance was actually completed and signed.
- The Flagged Justification: If the rules are met, the system doesn’t hide the note. Instead, it flags it as: “Reviewed Exception – Justification Requires Confirmation”.
The QA reviewer is directed straight to this flag. Their job isn’t to find the needle; it is to verify that the “needle” the system found is acceptable based on the evidence provided.
When that same inspector asks, “Show me where this was evaluated,” the site head no longer relies on a general statement about “careful reviewers.” Instead, they show the system audit trail: a digital flag, the linked SOP rule that authorized it, and the QA electronic signature confirming the justification was verified.
One useful way to think about Review by Exception is through the same quality lens we already apply elsewhere. We routinely apply FMEA and QbD to almost everything in manufacturing be it equipment, processes, even the way materials move through a facility. Batch review is a bit of an exception. It is one of the few critical activities where the design assumption has quietly been: ‘a careful human will read everything.’ As batch records have grown longer and more complex, that assumption has become harder to defend. Review by Exception simply applies the same QbD thinking we already trust elsewhere, defining what truly matters, designing controls around those risks, and allowing human judgment to focus where it’s most valuable.
How Mareana Implements Review by Exception to Speed Up Batch Release
The Mareana platform transforms paper-based chaos into a streamlined, digital intelligence hub using three core pillars:
1. AI-OCR: Turning Paper into Actionable Data
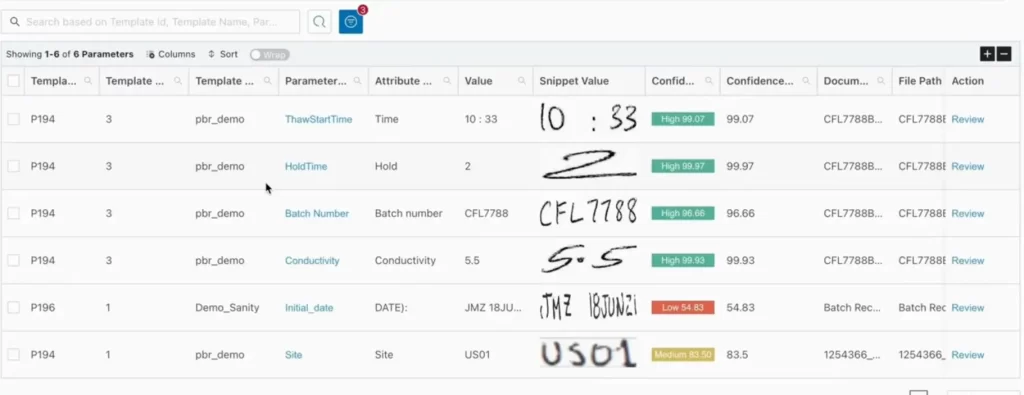
Before you can review by exception, you need digital data. Mareana’s AI-powered OCR (Optical Character Recognition) digitizes everything from handwritten signatures to complex tables.
- Automatic Classification: The platform identifies the document type and associates it with the correct Master Batch Record (MBR) template.
- Confidence Scoring: The system uses “confidence levels” (Green, Yellow, Red) to indicate how accurately it read the data. Only low-confidence “snippets” require a human-in-the-loop for verification, drastically reducing manual effort of reviewing.
2. Automated Rule Verification
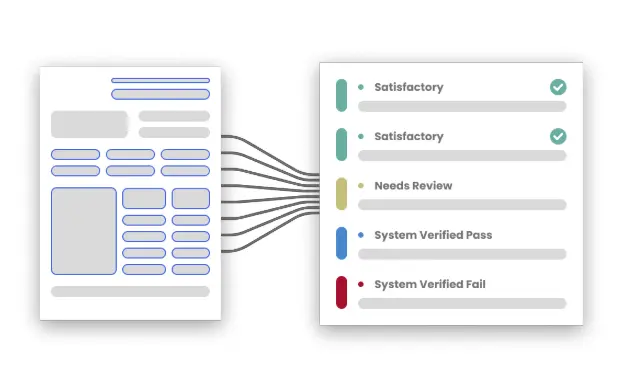
Mareana applies a “hard” rule-based engine to every digitized parameter to catch deviations instantly:
- System Verified Passes: Values within range and correct calculations are marked green and essentially accepted by the reviewer.
- Range & Calculation Checklist: Mareana performs real-time math verifications, even across multiple pages or separate documents to ensure consistency.
- Missing Value Detection: If an operator forgets a signature or leaves a field blank, the system flags it as a “fail” immediately.
3. AI Agents for Unstructured Exceptions
Not every error is a number out of range. Mareana uses specialized AI Agents to detect the “messy” parts of paper records:
- Margin Notes & Strikeouts: These variable notes often hide critical context but are easily missed by humans during a long shift.
- Anomaly Flagging: Agents identify and highlight these unstructured notes for the reviewer, ensuring nothing is buried in the fine print.
The Benefits: Why Pharma Leaders are Switching to Mareana
Implementing Review By Exception with the Mareana platform isn’t just about going digital; it’s about measurable efficiency:
- 70% Reduction in Review Time: By automating 85% of the routine checking, Mareana helps teams accelerate their batch release process by up to 70%.
- Increased Right-First-Time (RFT): Automated checks prevent “fatigue-driven” human errors from slipping through to final release.
- GxP by Design: The platform is fully validated and generates a complete, visual Batch Genealogy (Knowledge Graph), providing an immutable audit trail for regulators.
Final Thoughts
Manual review is riskier than you think because of many reasons, such as
- ReviewerFatigue: When reviewers deal with a high volume of batch parameters, they often get fatigued and prone to missing critical errors.
- Limit of Human Attention: A single batch record can contain hundreds of parameters; expecting a human to maintain 100% accuracy across every line of every page is statistically unlikely.
- Mathematical Human Error: Even simple calculations like subtracting tare weight from gross weight are frequently done incorrectly on the shop floor, and these errors can easily be overlooked during a manual audit.
- The “Invisible” Missing Data: Manual reviewers are tasked with finding what isn’t there, such as a missing signature or a blank field, which is much harder for the human eye to detect than an incorrect value.
- Unstructured Data Blindness: Margin notes, strikeouts, and informal corrections are variable and unpredictable; without an AI agent specifically looking for them, these critical pieces of context are often missed.
In an industry where a single day of delay can cost millions, Review by Exception is a competitive necessity. By using the Mareana Manufacturing Intelligence Platform, pharma manufacturers can stop “looking for needles in haystacks” and start releasing life-saving products faster and more confidently than ever before “Right now the blog explains how the system works. It needs to explain why trusting the current manual process is riskier than leaders think.”
Frequently Asked Questions (FAQs)
- What is Review by Exception (RbE) in pharmaceutical manufacturing?
Review by Exception is a QA approach where reviewers focus only on data that deviates from predefined rules or expectations. Instead of rereading every page, QA teams review system-flagged exceptions, ensuring expertise is applied where risk actually exists. - Why is manual batch record review considered risky today?
Manual review relies on sustained human attention across hundreds of pages and parameters. Fatigue, time pressure, and cognitive overload make it statistically likely that errors—especially missing data or contextual notes—will be overlooked, even by experienced reviewers. - How does AI make Review by Exception possible now when OCR failed before?
Modern AI can accurately read handwritten text, understand context, and apply validated logic. Unlike legacy OCR, today’s systems assess not just legibility but correctness, confidence levels, calculations, and rule compliance—dramatically reducing false positives and rework. - How does RbE help during regulatory inspections?
RbE creates a clear digital audit trail showing exactly what was reviewed, why it was flagged, which SOP or rule applied, and who approved it. This replaces vague assurances like “the reviewer checked it” with concrete, defensible evidence. - What types of issues does Review by Exception catch better than humans?
RbE excels at detecting:- Missing signatures or blank fields
- Calculation errors across pages
- Out-of-range or borderline values
- Handwritten margin notes and strikeouts
- Contextual deviations that meet limits but require justification
- These are often the most inspection-critical issues—and the easiest for humans to miss.
- Does Review by Exception remove human judgment from QA?
No. RbE amplifies human judgment. The system finds and structures the risk; the QA reviewer confirms acceptability based on evidence and intent. Humans stop searching for needles and start validating what truly matters. - What are the measurable benefits of adopting Review by Exception?
Organizations implementing RbE typically see:- Up to 70% reduction in batch review time
- Higher Right-First-Time (RFT) rates
- Faster batch release and reduced inventory hold costs
- Stronger, more defensible GxP compliance
- Why is trusting the current manual process riskier than leaders think?
Because the process assumes a “careful human will read everything,” an assumption that no longer holds at modern scale. As batch records grow longer and more complex, the probability of missed errors increases—while AI-assisted RbE consistently applies the same rules, every time, without fatigue.




 Learn more
Learn more