The Quality Assurance teams involved in pharmaceutical manufacturing carry the critical responsibility of ensuring product integrity, regulatory compliance, and patient safety. Each of these responsibilities depends on the ability to trace materials and processes accurately across the entire production lifecycle. Without complete and reliable traceability, it is difficult to verify that quality standards have been met or to respond effectively when issues arise. When a batch issue occurs in absence of a robust tracking process, finding the root cause or identifying affected products can be difficult and slow. Regulatory inspections demand complete traceability, but manual methods make it hard to retrieve accurate data quickly. In the case of a recall, manufacturers can over or underestimate the number of affected products, leading to serious consequences. Sophisticated pharmaceutical manufacturers use Genealogy to overcome such challenges, but many early-stage businesses still rely on paper-based, manual processes. This blog post aims to explain genealogy to quality assurance leaders who are exploring it for their business. We’ll also discuss the role of Artificial Intelligence in modern genealogy setups.
What is Genealogy?
Product genealogy, also known as manufacturing or material genealogy, refers to the detailed history and lineage of a product throughout its manufacturing journey. It tracks everything from the raw materials used, through all stages of the manufacturing process, to the finished product, including its subsequent distribution. This meticulous record includes information about the raw materials, components, equipment utilized, inspection results, lot and serial numbers, dates, and quantities involved in production. For a Quality Assurance leader, product genealogy is crucial because it provides a comprehensive, documented audit trail that ensures every part of a product’s journey is accounted for.
What problems does Genealogy solve?
Here are a few examples highlighting the benefits of genealogy
- Accelerates investigations: Genealogy enables quick tracing of raw materials, intermediates, and equipment involved in a batch, significantly reducing the time needed to investigate deviations or non-conformances.
- Improves recall precision: With full traceability, QA teams can identify exactly which batches are impacted.
- Ensures audit readiness: Comprehensive batch genealogy provides documented evidence of material flow and process compliance, making it easier to respond to regulatory audits and inspections.
- Supports change impact analysis: When a material, supplier, or process changes, genealogy helps assess which products may be affected, reducing the risk of quality issues going unnoticed.
- Reduces manual errors: Automated traceability minimizes the dependency on paper records and spreadsheets, which are prone to human error and data gaps.
- Improves cross-functional alignment: Genealogy connects data across production (including CMOs and CDMOs), quality, and supply chain, giving QA a clearer view of the full manufacturing process.
- Detecting anomalies: Anomalies can be rare and unexpected, but they can indicate early signs of process shifts, equipment issues, or data inconsistencies. By analyzing historical and real-time data, Genealogy highlights these patterns so teams can investigate, troubleshoot, and take corrective action more effectively.
- Spotting Process Variations: Process variation occurs when a process no longer follows its expected pattern—due to factors like equipment wear, raw material inconsistency, or human error. Genealogy detects these variations in real time by continuously tracking how each step performs against historical norms.
The benefits of implementing Genealogy in Pharma Manufacturing Process
Genealogy brings structure and clarity to complex manufacturing data, delivering tangible benefits across operations:
- Increased Product Quality: By tracing every step, material, and parameter involved in production, Genealogy helps identify quality risks early and ensures consistent outputs.
- Ensured Compliance: With complete traceability and versioned process history, it supports audit readiness and simplifies regulatory reporting.
- Improved Predictability: By monitoring patterns across batches and sites, Genealogy enables early detection of process deviations—helping teams anticipate issues before they escalate.
- Enhanced Overall Efficiency: Teams spend less time compiling data and more time acting on insights, speeding up investigations and decision-making.
- Reduced Waste and Expenses: By catching issues early and improving process stability, Genealogy minimizes rework, scrap, and cost overruns.
The challenges with Genealogy
The benefits of genealogy are unquestionable, but many pharmaceutical manufacturers face practical challenges that delay or prevent its implementation. In many cases, these challenges are not due to a lack of need, but rather the complexity of putting a robust genealogy system in place. One of the main obstacles is the fragmentation of data across systems. Material movements, batch records, equipment logs, and test results often reside in separate platforms, or in some cases, locked inside filing cabinets. Integrating this data into a single, platform requires significant effort and system alignment. Additionally, legacy infrastructure in many manufacturing sites lacks the digital maturity needed to support real-time traceability. Cost and complexity also play a role. For smaller operations, the perceived investment in time, technology, and training, can seem too high compared to short-term benefits. Without a clear roadmap or leadership commitment, genealogy initiatives may stall or be deprioritized.
How Artificial Intelligence is making Genealogy easier to implement
AI is playing a growing role in making genealogy more accessible and effective for pharmaceutical manufacturers. For instance, at Mareana, our platform brings the full power of AI to manufacturing genealogy. Our AI-assisted batch release engine digitizes paper batch records with speed and accuracy; and feeds them directly into the genealogy in a structured form. Users can interact with the system through natural language via our AI chatbot, and ask questions about batches, materials, or process deviations without the need to navigate complex systems. It’s like talking directly to your manufacturing history. By integrating data from LIMS, QMS, CDMOs, and other sources, Mareana’s AI-powered genealogy shortens investigation times and simplifies root cause analysis. What once took days can now be done in minutes, that too with full traceability and confidence.
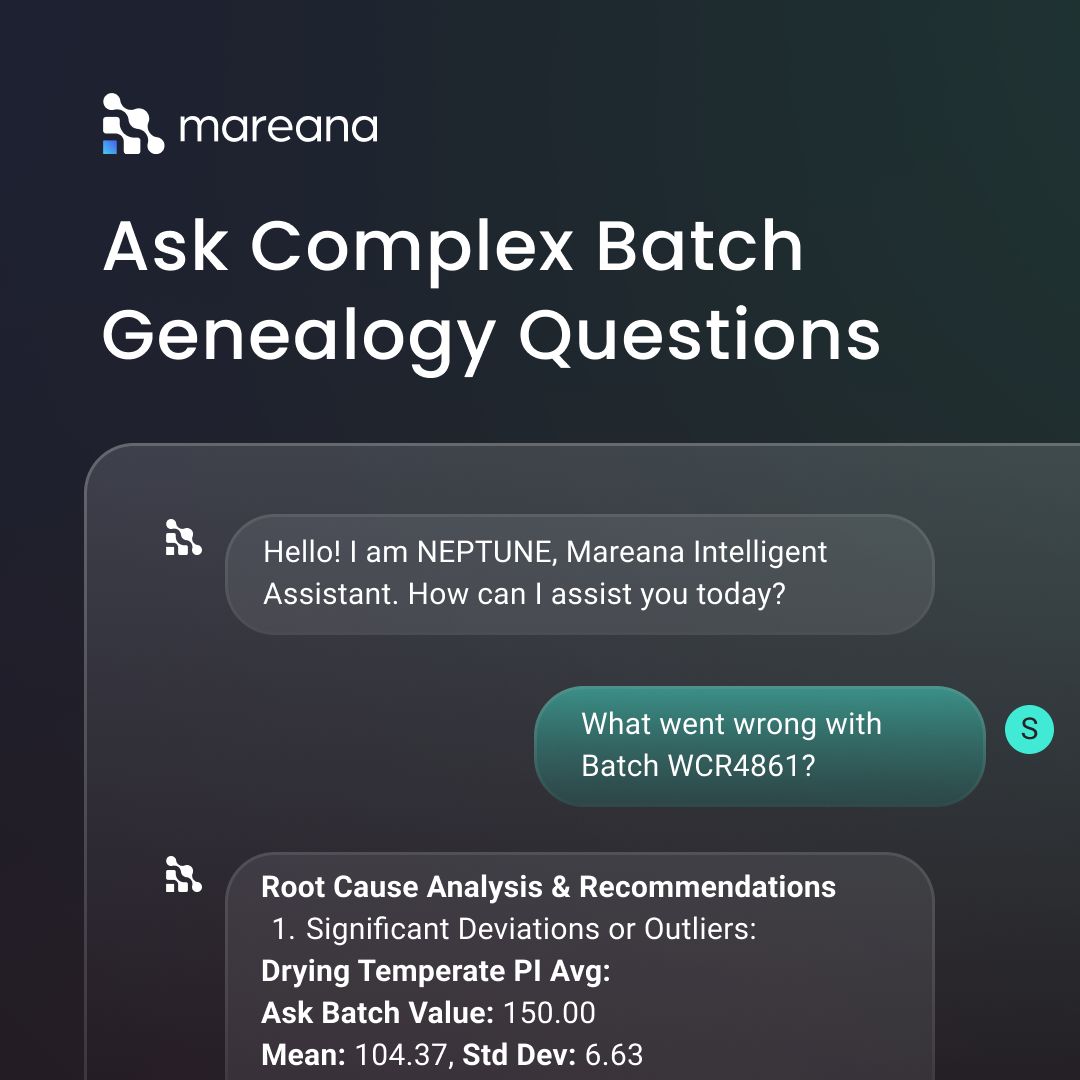
What’s Next?
If you are a QA leader and you want to improve traceability in your manufacturing process, implementing genealogy can be a very impactful initiative. Do you want to see Genealogy in action? You can book a demo with us. And finally, if you want to learn more about how Artificial Intelligence is being used with Genealogy, read our next blog: How Artificial Intelligence Accelerates Product Genealogy Related read Revolutionizing Batch Release: Leveraging AI to enhance compliance and streamline operations in manufacturing
Frequently Asked Questions (FAQs)
-
What is product genealogy in pharmaceutical manufacturing?
Product genealogy refers to the detailed history and lineage of a pharmaceutical product throughout its manufacturing process. It records every material, process step, piece of equipment, and test result involved in production, ensuring full traceability and compliance with regulatory standards. -
Why is genealogy important for Quality Assurance teams?
Genealogy enables Quality Assurance (QA) teams to maintain complete traceability across production, helping verify product quality, respond quickly to deviations, and support regulatory audits. It provides a documented audit trail that ensures product integrity and patient safety throughout the lifecycle. -
What problems does genealogy solve in pharma manufacturing?
Genealogy solves several major challenges:- Accelerates investigations and root cause analysis
- Improves recall accuracy
- Ensures audit readiness and compliance
- Supports change impact analysis
- Detects anomalies and process variations
- Reduces manual errors from paper-based systems
-
How does genealogy improve product quality and compliance?
By connecting data from materials, equipment, and processes, genealogy identifies early signs of variation or risk. This enables proactive quality management, ensuring that deviations are detected and corrected before they affect product safety or compliance with FDA and GMP standards. -
What are the main benefits of implementing genealogy in pharma manufacturing?
Implementing genealogy offers several key advantages:- Higher product quality through complete traceability
- Improved compliance and simplified audit preparation
- Predictive insights for early issue detection
- Faster investigations and decision-making
- Reduced waste and operational costs through better process control
-
What challenges do manufacturers face in implementing genealogy?
Common challenges include fragmented data across multiple systems (LIMS, QMS, ERP), lack of digital maturity in legacy infrastructure, and the perceived cost or complexity of integration. These factors can delay or discourage the implementation of comprehensive genealogy systems. -
How is Artificial Intelligence transforming genealogy implementation?
AI simplifies genealogy by automating data digitization, integration, and analysis. It converts paper batch records into structured data, builds real-time traceability maps, and connects information from diverse systems like LIMS, QMS, and CDMOs — making genealogy faster, smarter, and easier to maintain. -
What role does Mareana’s AI-powered platform play in genealogy?
Mareana’s AI-driven platform uses intelligent data extraction and natural language processing to build and manage digital genealogy. Through its AI chatbot, users can query batch data, materials, or deviations conversationally — drastically reducing investigation time and improving decision-making accuracy. -
How does AI improve investigation and root cause analysis in genealogy?
AI rapidly analyzes large datasets from multiple systems to detect trends, anomalies, or process variations. It highlights possible root causes and provides actionable insights, cutting investigation times from days to minutes while ensuring full traceability and data integrity. -
Why should early-stage pharmaceutical companies adopt AI-driven genealogy?
For growing manufacturers, implementing AI-enabled genealogy offers long-term benefits such as:- Reduced manual effort and data errors
- Scalable, compliant traceability systems
- Enhanced visibility across CMOs and CDMOs
- Faster regulatory response and audit readiness
- Stronger product quality assurance from the outset



 Learn more
Learn more


