Good Manufacturing Practices (GMP), often referred to as Current Good Manufacturing Practice (CGMP) by the U.S. Food and Drug Administration (FDA), represent the minimum standards that manufacturers must meet in their production processes to ensure product quality. The FDA’s CGMP regulations provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. A critical aspect of the FDA’s CGMP regulations is the “Current” (C) in CGMP. This signifies that manufacturers are required to employ technologies and systems that are up-to-date. This flexibility is not merely an allowance but an encouragement for companies to utilize modern technologies and innovative approaches to achieve higher quality through continual improvement. This regulatory stance is a crucial enabler for the adoption of advanced technologies like AI, as it provides a clear pathway for integrating modern solutions into GMP-compliant operations. Therefore, in this blog post, we’ll discuss how Artificial Intelligence is being used by Quality Assurance Professionals for GMP adherence. We’ll also provide you some real-world examples to help you explore the opportunities to improve manufacturing processes in your facilities.
Batch release and product quality reviews through data analysis and chatbots
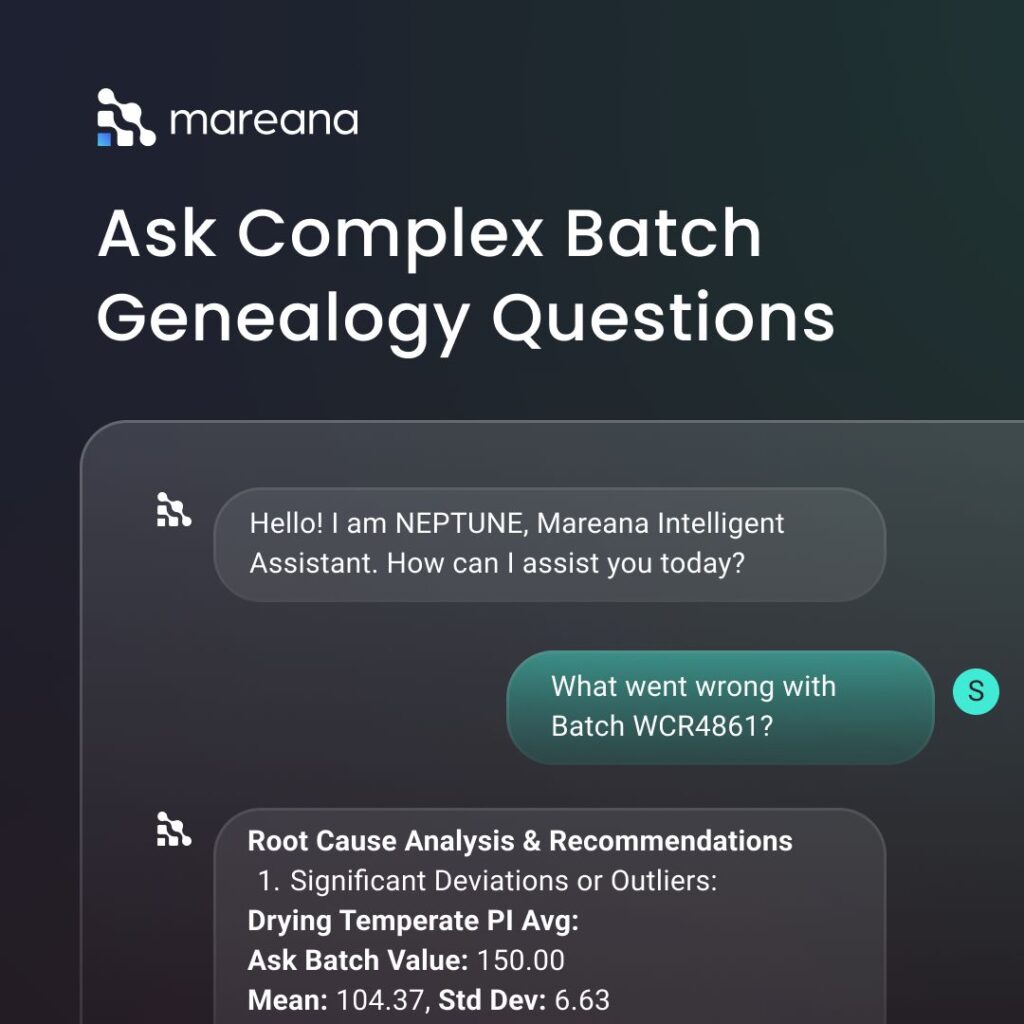
AI can automatically collect, aggregate, and cross-reference data from various source systems (PAT, MES, LIMS, ERP, QMS, environmental monitoring) relevant to a batch. By analyzing historical batch data and in-process controls, it can do a real-time release prediction. This enables RTR based or conditional release scenarios. For annual product quality reviews (APQR), AI can analyze years of batch data, complaint records, stability studies, etc., to identify long-term trends, subtle shifts in quality attributes, and emerging risks that might be missed manually. AI provides comprehensive data summaries and risk insights to quality professionals, supporting faster and more confident batch release decisions. Organizations move to a “single click APQR with the aid of AI. Mareana’s Neptune chatbot is a great example of how AI is helping batch reviews and batch release to improve GMP adherence. The chatbot can analyze the genealogy of the product and answer questions in natural language. Quality professionals can review batches with simple prompt like “How many batches were produced using Lot 2354318 of Purified Water?”. This AI-capability shortens investigation times and simplifies batch reviews.
Deviation Management though anomaly detection algorithms and real-time alerts
AI improves deviation management by enabling real-time detection, intelligent root cause analysis, predictive recurrence prevention, and automated workflow. AI algorithms connected to sensors and manufacturing equipment can detect subtle anomalies or trends in process parameters that indicate a deviation before it becomes significant. This enables proactive intervention. Upon detection, AI can automatically classify the deviation based on its type, severity, and potential impact on product quality, guiding immediate prioritization. An example of AI-enabled deviation management is Mareana’s Continuous Process Verification (CPV/OPV). It applies advanced analytics and anomaly detection algorithms to identify deviations in real time and trigger alerts for immediate corrective action, ensuring consistent product quality. AI-enables deviation management is transforming the traditional CPV/OPV programs in a powerful real-time monitoring and improvement program.
Risk assessment through virtual environment simulation
AI transforms GMP risk assessment from a subjective, manual process into a data-driven, predictive, and continuous activity. It analyzes vast historical and real-time data to identify subtle patterns, predict potential issues, and quantify risks. This allows companies to prioritize resources and implement proactive mitigation strategies. An example of AI-powered risk assessment is Mareana’s No Code ML (NCML) or Data Science Studio, where the platform can predict the impact of even the slightest variations in Critical quality attributes and Critical quality parameters. Manufacturers can try various permutations and combinations in a virtual environment to simulate outcomes, identify potential risks, and optimize processes—without running costly physical trials. This not only accelerates decision-making but also enhances product quality, ensures compliance, and minimizes the likelihood of failures or deviations during actual production. Manufacturers can roll out these DSS/NCML templates across multiple production lines/sites to monitor these parameters in a real-time.
Supply chain quality control by monitoring knowledge graphs
AI is capable to extend GMP oversight beyond the manufacturing facility, providing greater visibility, traceability, and risk management across the complex pharmaceutical supply chain. It can be used to analyzes supplier performance data, audit results, regulatory history, etc. to assess and predict supplier quality risks. In cases of supply chain disruptions, it can recommend alternative sourcing or logistics strategies. An example of AI-enabled supply chain management is Mareana’s Value Stream Mapping (VSM) capability. AI algorithms detect products, sites, lanes, and their parent-child links to create an end-to-end supply chain knowledge graph. The system continuously calculates lead times, cycle times, OTIF, inventory health, slow/obsolete stock, and back-order exposure. Visual tools like map views, heat maps, and drill-downs enable planners to trace any item forward or backward within seconds.
What’s Next?
AI is improving every stage of the manufacturing process and helping business follow GMPs more effectively. If you’re considering AI for your pharmaceutical manufacturing workflow but aren’t sure where to start, feel free to contact us.
Frequently Asked Questions (FAQs)
1. What are Good Manufacturing Practices (GMP) and why are they important?
Good Manufacturing Practices (GMP), or Current Good Manufacturing Practice (CGMP) as defined by the FDA, are a set of regulations that ensure products are consistently produced and controlled according to quality standards. They protect consumers by minimizing risks such as contamination, mix-ups, and product defects.
2. What does the “Current” in CGMP mean?
The “Current” in CGMP signifies that manufacturers are expected to use up-to-date technologies and modern quality systems. It encourages the adoption of innovative tools like Artificial Intelligence (AI) to enhance product quality, improve monitoring, and promote continuous improvement in manufacturing.
3. How is Artificial Intelligence being used for GMP adherence?
AI supports GMP adherence by automating data collection, analysis, and decision-making processes. It helps in batch release, deviation management, risk assessment, and supply chain monitoring—allowing manufacturers to maintain compliance while improving efficiency and product quality.
4. How does AI improve batch release and product quality reviews?
AI can analyze data from multiple systems such as LIMS, MES, QMS, ERP, and environmental monitoring tools to predict batch outcomes in real time. It supports real-time release (RTR) decisions and streamlines Annual Product Quality Reviews (APQR) by identifying long-term trends and potential risks with a “single-click” summary.
5. What role does Mareana’s Neptune chatbot play in GMP compliance?
Mareana’s Neptune chatbot enables quality professionals to interact with manufacturing data through natural language. It can answer complex batch-related questions instantly—such as tracing genealogy or identifying materials used—significantly reducing review and investigation time while improving data accuracy and traceability.
6. How does AI enhance deviation management in pharmaceutical manufacturing?
AI uses anomaly detection algorithms and real-time analytics to identify process deviations before they impact product quality. Platforms like Mareana’s Continuous Process Verification (CPV/OPV) apply machine learning to classify and prioritize deviations, ensuring faster corrective actions and preventing recurrence.
7. In what ways can AI improve GMP risk assessment?
AI transforms traditional risk assessments into continuous, predictive processes. By analyzing large volumes of historical and live data, it identifies patterns and quantifies risks. Tools like Mareana’s Data Science Studio (DSS) simulate outcomes in a virtual environment, enabling proactive decision-making without physical experimentation.
8. How does AI support supply chain quality control under GMP?
AI provides end-to-end visibility of the pharmaceutical supply chain. It analyzes supplier data, regulatory records, and logistics information to identify potential risks. Using knowledge graphs and Value Stream Mapping (VSM), AI helps manufacturers trace products, predict disruptions, and optimize sourcing strategies.
9. How can manufacturers start integrating AI into GMP processes?
To begin, manufacturers should assess their current quality systems and identify key areas—like batch review, deviation tracking, and supplier management—that would benefit from automation. Partnering with an AI-driven manufacturing intelligence platform like Mareana ensures secure, compliant, and scalable implementation aligned with FDA expectations.
10. What are the main benefits of using AI for GMP compliance?
AI-driven GMP solutions provide multiple advantages:
- Enhanced data accuracy and integrity
- Faster investigations and batch releases
- Predictive risk management
- Stronger supplier oversight
- Reduced manual effort and compliance burden
- Continuous process improvement across production lines




 Learn more
Learn more


