Summary: Regulations like cGMP, ALCOA++ principles, and 21 CFR Part 11 demand strict data integrity, traceability, and digital audit readiness. Mareana’s Manufacturing Intelligence Platform leverages AI, OCR, and knowledge graphs to digitize records, unify data, and ensure compliance.
Why Compliance Is Non-Negotiable in Pharma
Every drug that reaches patients must meet the highest standards of quality and integrity. Regulators, especially the U.S. Food and Drug Administration (FDA), scrutinize every process, document, and data point to ensure public health is never compromised.
But here’s the catch: keeping up with evolving compliance requirements is challenging. Manual, paper-heavy processes, data silos, and fragmented systems often stand in the way of smooth compliance. That’s where Mareana’s Manufacturing Intelligence Platform steps in—transforming compliance into a proactive driver of operational excellence.
Understanding the Regulatory Landscape in Pharma Manufacturing
The importance of cGMP
The backbone of pharma regulation lies in Current Good Manufacturing Practice (cGMP). These rules ensure that medicines are consistently produced under strict quality standards.
The FDA expects manufacturers to maintain systems that assure proper design, monitoring, and control of manufacturing processes. Mareana helps unify data across systems into one accessible platform, making outcomes compliant and transparent.
Data Integrity and ALCOA++ Prinicples
Data integrity is the foundation of regulatory compliance in pharma manufacturing. The ALCOA++ principles—Attributable, Legible, Contemporaneous, Original, Accurate, Complete, Consistent, Enduring, Available, Traceable—guide how pharma companies must handle data.
For a robust implementation of ALCOA++ principles, Mareana leverages pharma-specific AI-OCR to convert unstructured, error-prone paper records into digital, auditable formats during its Paper Batch Record Digitization process. This ensures compliance with ALCOA++ while providing regulators with an immutable trail of every action. Additionally, you can use this digitized data to obtain better insights about your manufacturing process.
Watch this video for more insights on paper batch record digitization with our AI-powered co-pilot
Transitioning to Digital with 21 CFR Part 11
As pharma embraces digital transformation, 21 CFR Part 11 governs the use of electronic records and signatures. It requires system validation, audit trails, security, and user permissions to ensure digital records are as reliable as paper-based ones.
Mareana automatically creates a 21 CFR Part 11-compliant audit trail, ensuring data integrity and minimizing audit risks. By consolidating data into one platform, it also makes inspections smoother and faster. This is done with the help of ai-powered knowledge graph technology which helps you create product genealogy, a detailed history and lineage of a product throughout its manufacturing journey.
Here is a glimpse of product genealogy in action.
Continuous Improvement with CAPA & Batch Release
The FDA requires a strong Corrective and Preventive Action (CAPA) process to address problems at their root. Unfortunately, many manufacturers struggle with deficiencies here, leading to warning letters and delays.
Mareana’s Batch Release Copilot reduces batch review time by up to 70%, while its Neptune AI chatbot accelerates investigations. Together, they simplify CAPA, speed up product release, and ensure compliance at every step.
Neptune AI chatbot is the future of pharma investigations. With the help of this generative AI capability, you can ask batch-related questions in natural language and get the answers instantly. There is no need of manually navigating through any kind of data management software to find the information you need.
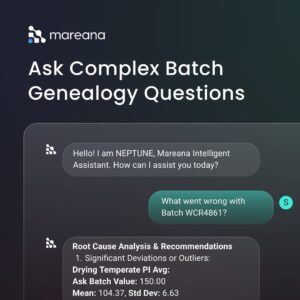
And there is a lot more. Read our white paper: How Mareana supports regulatory submissions in life sciences.
Summing Up
What the FDA Expects from Manufacturers:
- End-to-End Data Trail: Inspectors follow data from raw material to final product.
- Validated Systems: Every system must prove it works as intended.
- Robust Documentation: Every action must be attributable and traceable.
- Proactive Quality Control: Issues must be investigated at the root, not patched reactively.
Mareana’s platform ensures all of these expectations are not only met, but exceeded. In an industry that still relies heavily on paper, answering an FDA question about the source of a value in a report can be reduced to just a few clicks in MI.
The Biggest Challenges in Pharma Compliance:
- Manual, Paper-Based Processes: prone to human errors and inconsistencies.
- Siloed Data: makes it hard to get a complete view of operations.
- Slow Batch Release: delays time-to-market and increases compliance risk.
How Mareana empowers Pharma Manufacturers:
- Digitization of Paper Records for ALCOA++ – ensures reliable, traceable digital data.
- Always-On Audit Trails and Traceability – simplifies inspections with an immutable data trail.
- AI-Driven Batch Release Copilot – reduces review time by 70%.
- Accelerating Root Cause Analysis with Neptune – turns days of investigation into minutes.
Conclusion
Traditionally, compliance has been seen as a costly burden. Mareana flips the script, turning compliance into a strategic advantage. By eliminating manual risks, unifying data, and accelerating workflows, it not only reduces compliance risks but also enhances operational efficiency and time-to-market.
Frequently Asked Questions (FAQs)
-
Why is compliance so critical in pharmaceutical manufacturing?
Compliance ensures patient safety, product quality, and adherence to regulatory standards set by bodies like the FDA. Non-compliance can lead to fines, product recalls, or even loss of manufacturing licenses. -
What role does cGMP play in pharma compliance?
Current Good Manufacturing Practice (cGMP) is the backbone of pharmaceutical regulation, requiring companies to design, monitor, and control processes that ensure consistent product quality and safety. -
How do ALCOA++ principles impact data integrity in pharma?
ALCOA++ principles ensure data is attributable, accurate, complete, consistent, and traceable. Mareana’s AI-OCR digitizes paper records, turning them into compliant, auditable digital data that regulators can trust. -
What is 21 CFR Part 11, and why does it matter?
21 CFR Part 11 governs electronic records and signatures in pharma. It ensures digital systems meet strict validation, security, and audit trail requirements, making them as reliable as paper-based systems. -
How does AI improve compliance processes in pharma?
AI enhances compliance by digitizing paper records, automating audit trails, generating product genealogy, and enabling natural-language queries through tools like Neptune AI, reducing errors and improving efficiency. -
How can AI support CAPA and batch release compliance?
Mareana’s Batch Release Copilot cuts review time by up to 70%, while the Neptune AI chatbot accelerates investigations, helping manufacturers address issues faster and release products without delays. -
Can AI-powered platforms reduce audit risks?
Yes. By unifying data and creating 21 CFR Part 11-compliant audit trails, AI-powered platforms like Mareana provide regulators with transparent, tamper-proof records, reducing the chance of compliance gaps during audits.




 Learn more
Learn more


