Your AI can predict protein folding with quantum precision. Your ERP system tracks every penny across seventeen countries. Your MES records 847 process parameters per second. Your QMS generates compliance reports that would make the FDA weep tears of joy.
So why, when the VP of Manufacturing asks “What happened with Batch #47291 last Tuesday?” does everyone suddenly start looking at their shoes?
The Pharmaceutical Digital Transformation Paradox: Millions in Sophisticated Confusion
We’ve spent the last decade building the most sophisticated pharmaceutical manufacturing infrastructure in human history. Pharmaceutical digital transformation has created platforms that generate more data per day than existed in all of human knowledge just fifty years ago.
And yet, when something goes wrong—when a batch fails, when yields drop, when an inspector asks a simple question—we find ourselves in the same place our predecessors were in 1992: hunting through paper trails, calling people who were on shift, and hoping someone remembers something useful.
This isn’t just a hypothetical problem. The FDA issued 190 warning letters to drug and biologics manufacturers in fiscal year 2024 alone—each one a $50M lesson in why FDA warning letter prevention isn’t about compliance software, it’s about manufacturing psychology. In a recent warning letter to a major pharmaceutical manufacturer, the FDA found that “production staff could alter master batch records. These included ‘modifying batch formulations and other parameters or formulations before printing.’”
The real issue? These companies had invested millions in pharmaceutical data integrity solutions, yet still couldn’t maintain basic data integrity across their manufacturing processes.
This isn’t a technology problem. It’s a psychology problem that pharmaceutical data integrity solutions have completely misunderstood.
The Comfortable Tyranny of Paper
Here’s what the consultants won’t tell you: humans trust paper because paper tells stories. Your batch record management software speaks database, but your manufacturing speaks relationships—and that linguistic mismatch costs you millions.
When your most experienced QA manager picks up a printed batch record, she’s not just reading data points. She’s reading the story of what happened. The slightly shaky signature that tells her the night shift operator was tired. The timing gap that reveals when the steam line acted up again. The handwritten note that explains why they had to deviate from standard procedure.

Paper is analog. Manufacturing is analog. People are analog.
Your digital systems, no matter how sophisticated, are digital. They capture what happened, but they don’t understand what it means. They record events, but they don’t reveal relationships. They store data, but they don’t preserve wisdom.
This is why your most experienced people still print things out. It’s not because they’re opposed to new technology or ways of working. It’s because they’re human, and humans have evolved to recognize patterns in narratives, not in databases.
The FDA Inspection Question That Costs Millions When You Can’t Answer It
Imagine this question during a routine FDA inspection: “I see these three batches all used raw material from the same supplier lot. Can you show me how you verified that this supplier lot met your enhanced specifications?”
Simple question. Any GxP manufacturing compliance software should answer inspector questions in 30 seconds—not 30 hours of digital archaeology.
How much time would your team take? Would it take minutes or hours?
The delay in response is not because they didn’t have the data. They had all the data. They had digital records in their QMS, batch genealogy in their MES, supplier qualifications in their ERP, and test results in their LIMS. They had so much data that finding the right data became impossible.
This scenario isn’t hypothetical—it’s playing out across the industry. Recent FDA warning letters reveal a pattern of companies struggling with basic data correlation. In one 2024 case, the FDA cited a manufacturer for failing to maintain adequate batch records, noting “missing identity and strength testing, an inadequate stability testing programme and—of course—‘Data Integrity Remediation.’ As a conclusion, FDA stated that the company’s ‘quality systems are inadequate’ and placed the firm on Import Alert.
The problem wasn’t missing information. The problem was missing connections.
Your digital systems don’t talk to each other in the language of manufacturing relationships. They talk to each other in the language of database queries. According to FDA’s 21 CFR Part 11 guidance on electronic records and signatures companies must ensure that their computerized systems provide “accurate and complete copy of records,” yet many organizations struggle to correlate records across multiple validated systems.
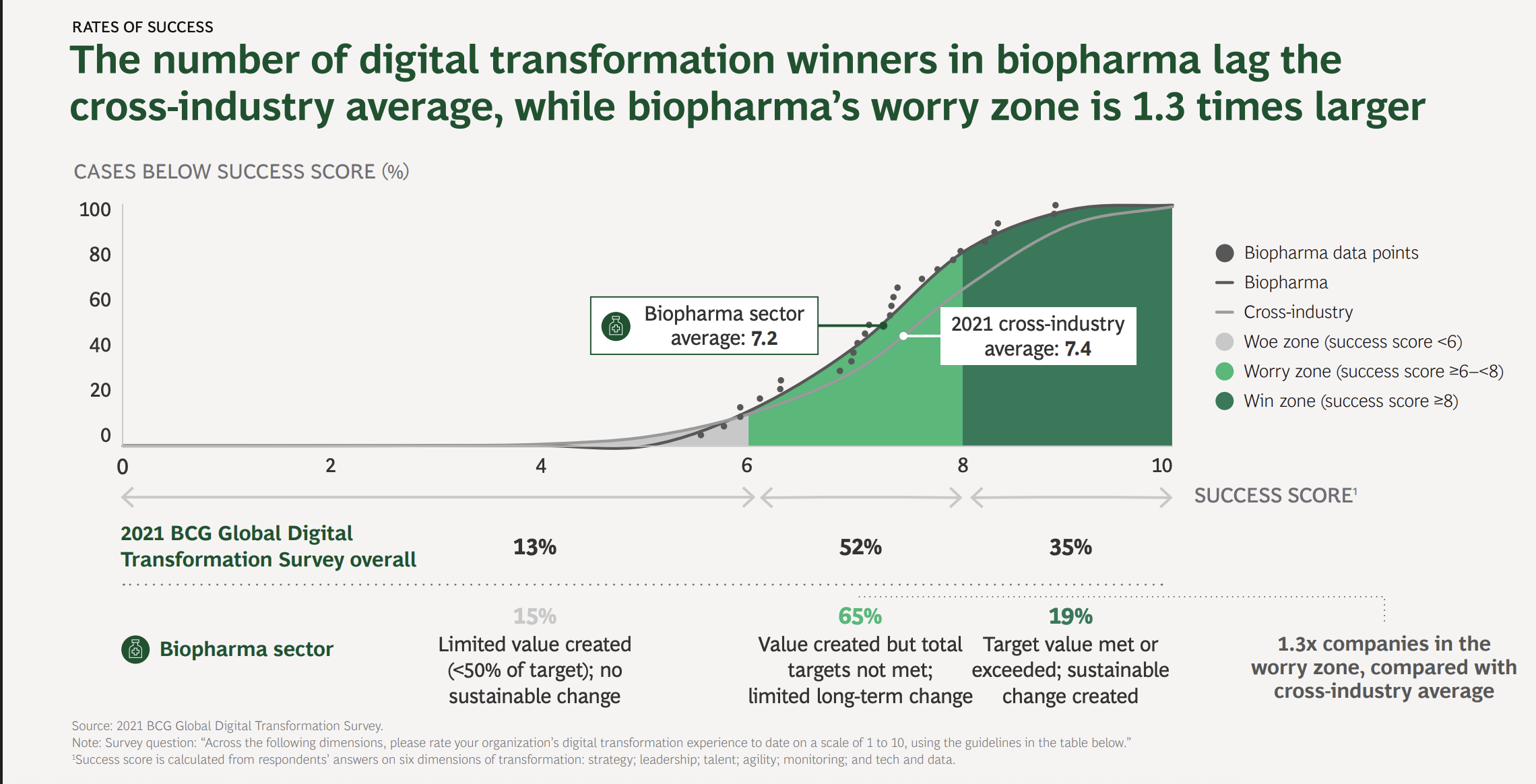
Industry data confirms this crisis: Why pharmaceutical digital transformation fails isn’t a mystery—it’s a predictable result of trying to solve human psychology problems with database solutions. Reports indicate that less than 20% of biopharma companies have successfully executed a digital transformation, while the cross-industry average is around 35%, according to a report by the Boston Consulting Group (BCG); companies are struggling with system integration more than any other sector.
When you need to understand how Batch A is related to Batch B, which is related to Raw Material C, which came from Supplier D, who was qualified based on Test E—your systems make you become a digital archaeologist.
You have to know which system contains which piece of information. You have to know how to query each system. You have to know how to correlate timestamps across systems that don’t share the same time source. You have to manually reconstruct the relationships that should be obvious.
The Psychology of Manufacturing Anxiety: When Pharmaceutical Manufacturing Compliance Creates Existential Dread
There’s a particular kind of stress that exists only in pharmaceutical manufacturing. Pharmaceutical manufacturing compliance creates a unique form of anxiety: the fear of not knowing what you don’t know—while lives depend on knowing everything.
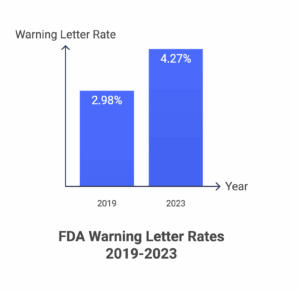
The regulatory pressure is intensifying. FDA inspection data shows warning letter rates increased from 2.98 per 100 inspections to 4.27 per 100 inspections between 2019-2023—a 43% increase. The most common violations? Data integrity issues and inadequate batch record systems.
People develop workarounds. They create shadow systems. They print things out and put them in binders “just in case.” They maintain Excel spreadsheets that cross-reference information across multiple official systems. They develop tribal knowledge about which system to trust for which type of information.
This isn’t inefficiency. This is intelligent adaptation to poorly designed information architecture.
Your employees aren’t resisting digital transformation. They’re compensating for digital transformation that doesn’t understand the psychology of manufacturing confidence.
The Missing Link: Why Pharmaceutical Manufacturing Analytics Miss the Most Important Data
Here’s what your current systems are missing: they don’t understand that manufacturing is fundamentally about relationships.
Every batch has parents—the raw materials, the equipment, the environment, the people, the procedures that created it. Every batch has siblings—other batches made with the same inputs, the same conditions, the same methods. Every batch has children—the products, the customers, the patients who depend on it.
These relationships aren’t just data points. They’re the story of how your manufacturing process actually works. They’re the context that transforms information into intelligence.
Real FDA citations prove this point. In recent warning letters, the agency repeatedly cites manufacturers for failing to understand batch relationships. One company was cited because they “provided no rationale for how the samples tested were representative of the potential for overall batch contamination.” The problem wasn’t individual data points—it was the failure to understand how those data points related to the broader manufacturing context.
When Batch #47291 fails, pharmaceutical batch failure analysis that can’t connect failures to their family trees isn’t analysis—it’s autopsy without genealogy.
Your current systems can tell you that Batch #47291 used Raw Material Lot #XYZ-789. But batch genealogy software should work like ancestry.com for manufacturing—but instead, it works like a broken family tree where nobody can find their relatives. They can tell you that Batch #47291 was produced on Line 3. But they can’t easily show you the genealogical relationship between all the batches produced on Line 3 during the same time period.
They capture the what, but they miss the who, when, where, why, and—most importantly—the how.
The Confidence Crisis
The real cost of this missing intelligence isn’t efficiency—though the efficiency costs are enormous. The real cost is confidence.
Industry research validates this confidence crisis. We are seeing business model changes led by the rise of patient-specific therapies and a deepening reliance on modern, digital manufacturing systems to improve operational efficiency and increase ROI. Cloud-based systems and real-world data fuelled by artificial intelligence are becoming essential, yet most companies lack the foundational data connectivity to leverage these advances.
When we can’t easily understand the relationships between our manufacturing elements, we can’t be confident about our decisions. When we can’t be confident about our decisions, we become conservative. When we become conservative, we slow down. When we slow down, our competition passes us by.
More importantly, when you can’t quickly and confidently answer questions about your manufacturing relationships, you can’t have intelligent conversations with regulators. Instead of discussing the sophisticated risk management and process understanding that should characterize modern pharmaceutical manufacturing, you find yourself scrambling to prove competence.
The inspector who asks about those three batches isn’t trying to catch our mistake. They’re trying to understand whether we understand our own manufacturing process well enough to ensure patient safety. When we cannot demonstrate our understanding quickly, we fail a test you didn’t even know we were taking.
The Human Cost of Digital Confusion
The most experienced manufacturing professionals are spending 40% of their time on digital archaeology instead of manufacturing intelligence. Your pharmaceutical quality management system has turned quality experts into digital archaeologists—excavating data instead of preventing problems. And regulatory affairs specialists are becoming system integrators instead of compliance strategists.

This isn’t just inefficient. It’s wasteful of the most valuable resource in pharmaceutical manufacturing: human expertise.
The industry data is stark: Recent FDA warning letters reveal that companies with the most sophisticated individual systems often have the worst data integration problems. System did not have sufficient controls to prohibit deletion and alteration of data as cited in FDA 21 CFR Part 211.68—this was the finding at a company that had invested in digital transformation over the previous five years.
The people who understand your manufacturing process at the deepest level—who can recognize patterns, who can predict problems, who can make intelligent decisions under uncertainty—are being forced to become amateur IT specialists just to access the information they need to do their actual jobs.
Meanwhile, IT specialists, who understand systems and data but aren’t experts in manufacturing, are being asked to solve manufacturing problems with technology solutions. This creates a dangerous gap where neither group can be fully effective. The ISPE GAMP 5 guidance emphasizes that successful computerized systems require collaboration between business and IT stakeholders to ensure “fitness for intended use.”
The Economic Reality: What This Actually Costs
The financial impact is measurable and significant. Recent industry analysis shows that pharmaceutical companies lose an average of 15-20% of potential manufacturing efficiency due to poor data integration. For a mid-size pharmaceutical manufacturer with $2 billion in annual revenue, this represents $300-400 million in lost productivity annually.
The regulatory costs are even more concerning. Companies that received FDA warning letters for data integrity issues in 2024 faced average remediation costs running into millions, plus lost revenue from delayed product launches and import alerts.
The competitive disadvantage compounds over time. Companies that can rapidly respond to regulatory inquiries, quickly identify process improvements, and confidently release batches are capturing market share from companies still struggling with basic data connectivity.
The Question That Changes Everything
Here’s the question that should keep pharmaceutical executives awake at night: “If we can’t quickly understand the relationships between our manufacturing elements, how can we be confident that we truly understand our manufacturing process?”
And here’s the follow-up question that should terrify them: “If we don’t truly understand our manufacturing process, how can we ensure that we’re consistently producing safe, effective medications?”
The regulatory trends confirm these concerns. The 7th ISPE Pharma 4.0 Survey conducted in 2023 found that while companies are investing heavily in digital technologies, most are struggling with the fundamental challenge of data integration and relationship understanding.
The answers to these questions reveal why the most successful pharmaceutical companies of the next decade won’t be the ones with the most advanced individual systems.
- They’ll be the ones whose pharmaceutical manufacturing analytics has mastered the ‘what’ and finally figured out the ‘so what’—systems that actually understand manufacturing relationships.
- They’ll be the ones who can answer the inspector’s question in thirty seconds instead of four days.
- They’ll be the ones whose people spend their time on manufacturing intelligence instead of digital archaeology.
- They’ll be the ones who transform their existing investments into actual intelligence, rather than replacing them with more sophisticated confusion.
- FDA inspection preparedness software that requires 4 days to answer a 30-second question isn’t preparedness—it’s performance art.
“You know those questions that make your QA team uncomfortable? The ones that require calling three departments and checking five systems? Bring them to us. We’ll discuss how Mareana can connect your existing Paper batch records, MES, QMS, ERP, and LIMS to provide answers in minutes, not days.”
Want to see how this would work with your actual Batch #47291? We’ll show you in a 30-minute demo using your real data – Let’s connect
In the next post, we’ll explore how artificial intelligence and batch genealogy are finally solving the manufacturing relationship problem—and why the solution isn’t about replacing your systems, but about making them intelligent for the first time. Subscribe to our newsletter to stay updated with our latest posts.




 Learn more
Learn more