Connect CMC™ is a state-of-the-art digital SAAS product for pharma manufacturing that innovatively addresses the unique needs of the industry. It streamlines and optimizes the entire CMC lifecycle, ensures compliance, boosts efficiency, and speeds up market entry.
Mareana
Connect
CMC™
GxP-Compliant
& Purpose-Built

Meet GxP requirements with our purpose-built and validated solution. With built-in data integrity measures, ensure the accuracy and reliability of data for analytics and reporting.
End-to-End
Data Traceability
Get end-to-end traceability of data from raw material to finished product attributes.
User-Friendly
Interface

Minimize training time and maximize productivity with the best-in-class design enabling ease of use.
Enhanced
Scalability

Scale effortlessly within a business with your growing data and process needs. Our cloud-based application supports both small-scale projects and large, multi-site operations.
Automated Data
Processing
Extract and contextualize data from paper batch records and other third-party data sources using AI & automation framework.
Enterprise Digital
CMC Solution

Automate data management & compliance reporting across the entire drug development cycle.
Connect CMC™ - A Step Ahead

Speed Up Technology Transfer
Facilitate rapid knowledge transfer and technology implementation through streamlined processes

Save Investigation Time
Improve resource utilization and save valuable time in CMC operation
Scale Effortlessly
Scale seamlessly and adapt to evolving requirements, new molecules and new data
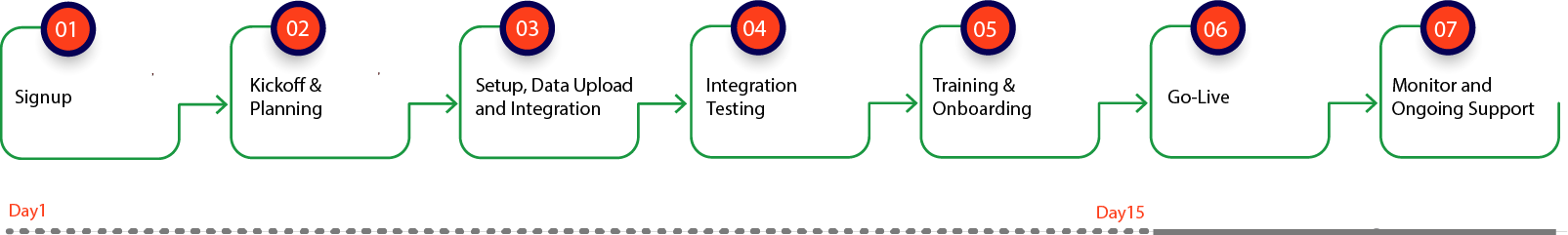
Implementation and Integration Steps
Answer: Connect CMC™ is a state-of-the-art digital CMC (Chemistry, Manufacturing, and Controls) platform designed for the pharmaceutical industry. It streamlines your manufacturing process, ensures compliance with regulatory standards, offers automated data integration from different source systems and of data types including paper batch records, data analytics with integrated data science studio, and simplifies reporting, ultimately enhancing efficiency and reducing time to market.
Answer: Our platform is built in accordance with global pharmaceutical standards, including FDA and EMA guidelines. It is fully validated and GMP compliant.
Answer: Currently the only data source we support are Paper batch records and soon we will have measures for seamless integration with a wide range of existing systems and platforms, ensuring minimal disruption to your operations.
Answer: Data security is a top priority for us. Connect CMC™ employs advanced security measures, including encryption and regular security audits, to ensure your data is protected at all times.
Answer: We offer comprehensive support including training sessions, a dedicated customer service team, and regular updates. Our goal is to ensure you maximize the benefits of Connect CMC™ in your operations.
Answer: Connect CMC™ stands out due to its comprehensive compliance management, Paper batch record data handling, integrated data analytics capability, user-friendly interface, and dedicated customer support. Our solution is specifically tailored to the needs of the pharmaceutical manufacturing industry, making it uniquely suited to address your challenges.
Answer: We provide extensive training for new users, including online tutorials, videos, and personalized training sessions, to ensure your team is fully equipped to use the product effectively.
Connect With Our Team
"*" indicates required fields




